- Like
- SHARE
- Digg
- Del
- Tumblr
- VKontakte
- Flattr
- Buffer
- Love This
- Save
- Odnoklassniki
- Meneame
- Blogger
- Amazon
- Yahoo Mail
- Gmail
- AOL
- Newsvine
- HackerNews
- Evernote
- MySpace
- Mail.ru
- Viadeo
- Line
- Comments
- Yummly
- SMS
- Viber
- Telegram
- JOIN
- Skype
- Facebook Messenger
- Kakao
- LiveJournal
- Yammer
- Edgar
- Fintel
- Mix
- Instapaper
- Copy Link
Introduction
Proteins are large molecules that perform many important functions in living organisms. One of these functions is to provide structure for cells and other tissues. To do this, proteins fold into an intricate 3D shape by following a specific pattern called the primary structure.
This process is aided by several different types of molecular interactions, including hydrogen bonding, ionic bonding, van der Waals forces, disulfide bridges, hydrophobic interactions, and more.
Unfortunately, when proteins misfold due to changes in their environment or genetic mutations, they can cause neurodegenerative diseases like Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, or amyotrophic lateral sclerosis (ALS).
What Are the Various Protein Structures?
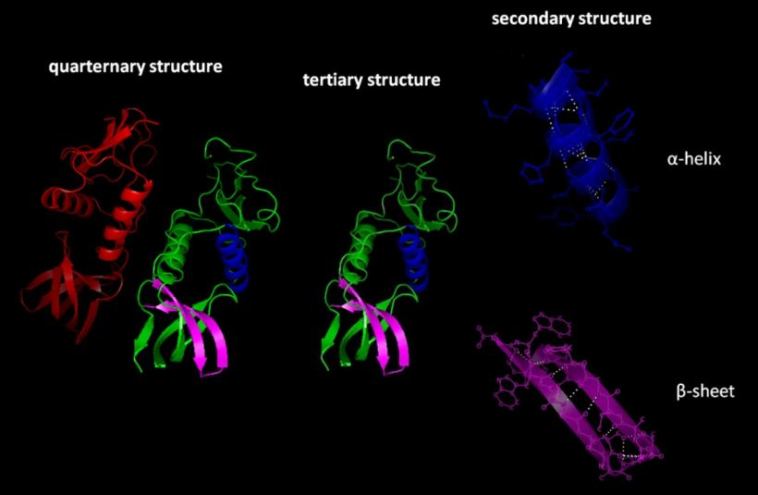
There are four main levels of protein structure: primary, secondary, tertiary, and quaternary. The primary structure is the linear sequence of amino acids that make up the protein.
Each sequence is unique to a specific type of protein and determines its properties (i.e. how it folds and what type of interactions it will form). The secondary structure is the first level of folding and refers to the local arrangement of amino acids into alpha helices and beta sheets.
Tertiary structure is a more complex form of folding that occurs when the secondary structures interact with one another to produce a final three-dimensional shape.
Finally, quaternary structure is the level of protein folding that occurs when multiple tertiary structures interact with each other.
How Does Protein Folding Occur?
Protein folding begins in the ribosome, where amino acid chains are synthesized. When it is time for a protein to be produced, these strings of amino acids detach from their transfer RNA (tRNA) molecules and fold into their proper conformation to create the primary structure.
This structural formation continues as neighboring proteins attach themselves through weak interactions as dictated by the tertiary structure, which can also briefly change as amino acids become covalently bonded through disulfide bridges and other interactions.
Why Is Protein Folding Important?
Protein folding is important because rare misfolded proteins called prions can lead to neurodegenerative diseases. Prions are proteins that can fold in multiple ways, and one of these conformations (termed the prion form) is infectious.
When a prion comes into contact with a properly folded protein, it can cause that protein to misfold and take on the prion form as well. This can create a chain reaction that causes large sections of the brain to become diseased.
What Happens When Misfolding Occurs?
When proteins misfold, they can clump together and form harmful plaques in the brain. These plaques destroy nerve cells and eventually lead to mental and physical disabilities, including impaired memory, tremors, difficulty walking or speaking, swallowing problems, muscle spasms/weakness, cognitive decline, and eventually, death.
Conclusion
Protein folding is a complex process that is important for the structure and function of cells. When proteins misfold, they can cause neurodegenerative diseases like Alzheimer’s, Parkinson’s, Huntington’s, and ALS.
Therefore, it is important to learn about these diseases and the role protein folding plays in them to better understand and treat them.
Sources: